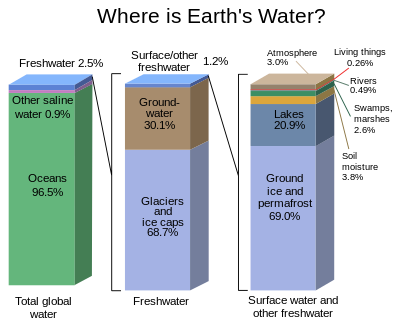
In the universe
Much of the universe's water is produced as a byproduct of star formation. When stars are born, their birth is accompanied by a strong outward wind of gas and dust. When this outflow of material eventually impacts the surrounding gas, the shock waves that are created compress and heat the gas. The water observed is quickly produced in this warm dense gas.
Water has been detected in interstellar clouds within our galaxy, the Milky Way. Water probably exists in abundance in other galaxies, too, because its components, hydrogen and oxygen, are among the most abundant elements in the universe. Interstellar clouds eventually condense into solar nebulae and solar systems such as ours.
Water vapor is present in
- Atmosphere of Mercury: 3.4%, and large amounts of water in Mercury's exosphere
- Atmosphere of Venus: 0.002%
- Earth's atmosphere: ~0.40% over full atmosphere, typically 1–4% at surface
- Atmosphere of Mars: 0.03%
- Atmosphere of Jupiter: 0.0004%
- Atmosphere of Saturn – in ices only
- Enceladus (moon of Saturn): 91%
- exoplanets known as HD 189733 b and HD 209458 b.
Liquid water is present on
Strong evidence suggests that liquid water is present just under the surface of Saturn's moon Enceladus. Jupiter's moon Europa may have liquid water in the form of a 100 km deep subsurface ocean, which would amount to more water than is in all the Earth's oceans.
Water ice is present on
- Earth – mainly as ice sheets
- polar ice caps on Mars
- Moon
- Titan
- Europa
- Saturn's rings
- Enceladus
- Pluto and Charon
- Comets and comet source populations (Kuiper belt and Oort cloud objects).
Water ice may be present on Ceres and Tethys. Water and other volatiles probably comprise much of the internal structures of Uranus and Neptune and the water in the deeper layers may be in the form of ionic water in which the molecules break down into a soup of hydrogen and oxygen ions, and deeper down as superionic water in which the oxygen crystallises but the hydrogen ions float around freely within the oxygen lattice.
Some of the Moon's minerals contain water molecules. For instance, in 2008 a laboratory device which ejects and identifies particles found small amounts of the compound in the inside of volcanic pearls brought from Moon to Earth by the Apollo 15 crew in 1971. NASA reported the detection of water molecules by NASA's Moon Mineralogy Mapper aboard the Indian Space Research Organization's Chandrayaan-1 spacecraft in September 2009.
Water and habitable zone
The existence of liquid water, and to a lesser extent its gaseous and solid forms, on Earth are vital to the existence of life on Earth as we know it. The Earth is located in the habitable zone of the solar system; if it were slightly closer to or farther from the Sun (about 5%, or about 8 million kilometers), the conditions which allow the three forms to be present simultaneously would be far less likely to exist.
Earth's gravity allows it to hold an atmosphere. Water vapor and carbon dioxide in the atmosphere provide a temperature buffer (greenhouse effect) which helps maintain a relatively steady surface temperature. If Earth were smaller, a thinner atmosphere would allow temperature extremes, thus preventing the accumulation of water except in polar ice caps (as on Mars).
The surface temperature of Earth has been relatively constant through geologic time despite varying levels of incoming solar radiation (insolation), indicating that a dynamic process governs Earth's temperature via a combination of greenhouse gases and surface or atmospheric albedo. This proposal is known as the Gaia hypothesis.
The state of water on a planet depends on ambient pressure, which is determined by the planet's gravity. If a planet is sufficiently massive, the water on it may be solid even at high temperatures, because of the high pressure caused by gravity, as it was observed on exoplanets Gliese 436 b and GJ 1214 b.
There are various theories about origin of water on Earth.